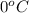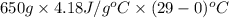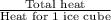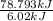Approximately how many ice cubes must melt to cool 650 milliliters of water from 29°C to 0°C? Assume that each ice cube contains 1 mole of H2O and is initially at 0°C. ∆H(fusion) = 6.02 kJ/mol; ∆H(vaporization) = 40.7 kJ/mol c(solid) = 2.09 J/g°C; c(liquid) = 4.18 J/g°C; c(gas) = 1.97 J/g°C Enter your answer numerically.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 1

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1
You know the right answer?
Approximately how many ice cubes must melt to cool 650 milliliters of water from 29°C to 0°C? Assume...
Questions


English, 14.10.2019 15:00

Mathematics, 14.10.2019 15:00


Mathematics, 14.10.2019 15:00



Mathematics, 14.10.2019 15:00

Chemistry, 14.10.2019 15:00

Business, 14.10.2019 15:00

Mathematics, 14.10.2019 15:00



History, 14.10.2019 15:00



Biology, 14.10.2019 15:00


Chemistry, 14.10.2019 15:00

Mathematics, 14.10.2019 15:00


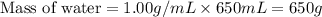
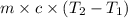

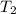 = final temperature =
= final temperature =