ASAP WILL GIVE BRAINLIEST
Write a balanced chemical equation for the following
chemical...

Chemistry, 22.04.2020 03:54 59bobolucci
ASAP WILL GIVE BRAINLIEST
Write a balanced chemical equation for the following
chemical reaction.
Solid magnesium reacts with aqueous copper (II) nitrate to form aqueous
magnesium nitrate and solid copper

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1


Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
Questions







English, 03.04.2020 17:28

Mathematics, 03.04.2020 17:28



Chemistry, 03.04.2020 17:29









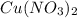 (aq) →
(aq) → 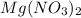 (aq) + Cu(s)
(aq) + Cu(s)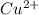 ) and nitrate (
) and nitrate (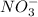 ). It will create:
). It will create: 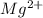 ) and nitrate (
) and nitrate (

