

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2


Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 23:30
If 2 molecules of one reactant combine with 5 molecules of another to produce 3 molecules of a product then which of the following is the correct representation of the reaction? a. 2a + 3b = 5c b. a2 + b5 = c3 c. a2 + b3 = c5 d. 2a + 5b = 3c
Answers: 3
You know the right answer?
A sample of air contains 78.08% nitrogen, 20.94% oxygen, 0.0500% carbon dioxide, and 0.930% argon by...
Questions





Mathematics, 31.10.2019 23:31


History, 31.10.2019 23:31


English, 31.10.2019 23:31

Mathematics, 31.10.2019 23:31

Mathematics, 31.10.2019 23:31



Mathematics, 31.10.2019 23:31

History, 31.10.2019 23:31

English, 31.10.2019 23:31


Mathematics, 31.10.2019 23:31

Biology, 31.10.2019 23:31

 ,
, 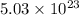 ,
,  and
and 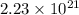 respectively.
respectively.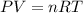
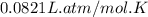
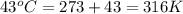
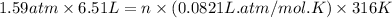
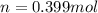
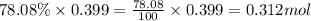
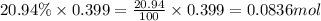
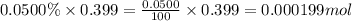
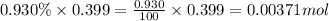
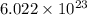 number of molecules of nitrogen.
number of molecules of nitrogen.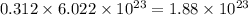 number of molecules of nitrogen.
number of molecules of nitrogen.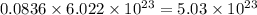 number of molecules of oxygen.
number of molecules of oxygen.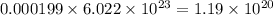 number of molecules of carbon dioxide.
number of molecules of carbon dioxide.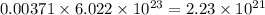 number of molecules of argon.
number of molecules of argon.

