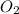13) Methane (CH4) combines with oxygen according to the balanced equation below. What is the limiting reactant if 25.0 g of CH4 combines with 50.0 g of O2? CH4 + 2 O2 → CO2 + 2 H2O moles CH4 moles O2 moles O2 needed to react with given amount of CH4 limiting reactant ("methane" or "oxygen")

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

You know the right answer?
13) Methane (CH4) combines with oxygen according to the balanced equation below. What is the limitin...
Questions

Physics, 20.09.2020 15:01

History, 20.09.2020 15:01

Mathematics, 20.09.2020 15:01

Chemistry, 20.09.2020 15:01



Computers and Technology, 20.09.2020 15:01

History, 20.09.2020 15:01



Mathematics, 20.09.2020 15:01


Physics, 20.09.2020 15:01



English, 20.09.2020 15:01


Biology, 20.09.2020 15:01


English, 20.09.2020 15:01