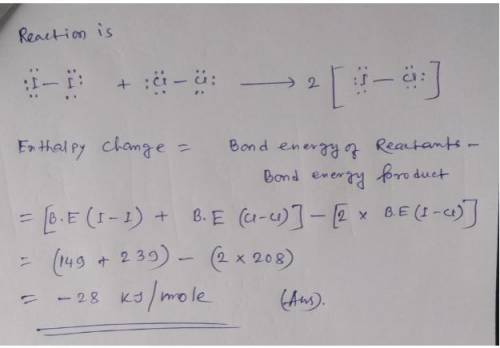Chemistry, 22.04.2020 04:27 tobyhollingsworth178
Calculate enthalpy of reaction using bond energies. Use the References to access important values if needed for this question. Use average bond enthalpies (linked above) to calculate the enthalpy change for the following gas-phase reaction. I2(g) +Cl2(g) 2ICI(g) To analyze the reaction, first draw Lewis structures for all reactant and product molecules. Include all valence lone pairs in your answer. Draw the reaction using separate sketchers for each species. One molecule per sketcher, please. Separate multiple reactants and/or products using the + sign from the drop-down arrow. Separate reactants from products using the symbol from the drop-down menu. If you have to draw H2, draw H-H.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
Calculate enthalpy of reaction using bond energies. Use the References to access important values if...
Questions








Computers and Technology, 23.09.2021 14:00

English, 23.09.2021 14:00

Mathematics, 23.09.2021 14:00

Mathematics, 23.09.2021 14:00



Mathematics, 23.09.2021 14:00

Chemistry, 23.09.2021 14:00

Mathematics, 23.09.2021 14:00


History, 23.09.2021 14:00

History, 23.09.2021 14:00