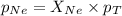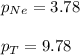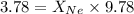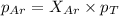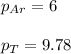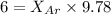Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 09:00
20 grams of water. she poured out 15 grams. which of the following physical properties of the water changes? a .boiling point b. density c .electrical conductivity d .volume
Answers: 2
You know the right answer?
A vessel containing Ne(g) and Ar(g) has a total pressure of 9.78. If the partial pressure of the Neo...
Questions





Biology, 21.11.2019 22:31


Social Studies, 21.11.2019 22:31

Biology, 21.11.2019 22:31




Biology, 21.11.2019 22:31








Social Studies, 21.11.2019 22:31

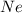 and
and 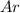 is, 0.387 and 0.613 respectively.
is, 0.387 and 0.613 respectively.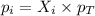
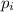 = partial pressure of gas
= partial pressure of gas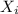 = mole fraction of gas
= mole fraction of gas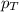 = total pressure of gas
= total pressure of gas