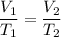Chemistry, 22.04.2020 05:28 twistedhyperboles
Calculate the temperature of a gas that originally occupied 3.45 L and is expanded to 5.25 L. The original temperature of the gas was 282K and the pressure remains constant

Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2

Chemistry, 23.06.2019 07:10
Which one of the following is an oxidation-reduction reaction? naoh + hno3 --> h2o + kno3 naoh + hno3 --> h2o + kno3 so3 + h2o --> h2so4 cacl2 + na2co3 --> caco3 + 2 nacl ch4 + 2 o2 --> co2 + 2 h2o al2(so4)3 + 6 koh --> 2 al(oh)3 + 3 k2so4
Answers: 3

You know the right answer?
Calculate the temperature of a gas that originally occupied 3.45 L and is expanded to 5.25 L. The or...
Questions

English, 23.05.2021 02:40








English, 23.05.2021 02:40

English, 23.05.2021 02:40

English, 23.05.2021 02:40

Biology, 23.05.2021 02:40


Mathematics, 23.05.2021 02:40

Mathematics, 23.05.2021 02:40

Mathematics, 23.05.2021 02:40