
Chemistry, 22.04.2020 16:34 isaiahmichel93081
A solution is prepared by dissolving 15.0 g of NH3 in 250 g of water. The density of the resulting solution is 0.974 g/mL. The mole fraction of NH3 in the solution is .A) 16.8B) 0.940C) 0.0597D) 0.922E) 0.0640

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

You know the right answer?
A solution is prepared by dissolving 15.0 g of NH3 in 250 g of water. The density of the resulting s...
Questions

Mathematics, 05.05.2020 00:45

Law, 05.05.2020 00:45





Mathematics, 05.05.2020 00:45

Spanish, 05.05.2020 00:45


Geography, 05.05.2020 00:45

Social Studies, 05.05.2020 00:45

Mathematics, 05.05.2020 00:45


Law, 05.05.2020 00:45

English, 05.05.2020 00:45


Chemistry, 05.05.2020 00:45

Computers and Technology, 05.05.2020 00:45

Mathematics, 05.05.2020 00:45

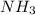 in solution is 0.0597.
in solution is 0.0597.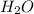 = 18.015 g/mol
= 18.015 g/mol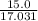 moles of
moles of 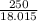 moles of
moles of 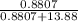 = 0.0597
= 0.0597

