For the balanced equation shown below, if the reaction of 0.112 grams of
H2 produces 0.7...

Chemistry, 22.04.2020 19:10 jjaerere6609
For the balanced equation shown below, if the reaction of 0.112 grams of
H2 produces 0.745 grams of H20, what is the percent yield? Fe3O4 + 4H2 -> 3Fe
+ 4H2O
74.5%
84.5%
94.5%
092.2%

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 23:00
Need asap question 1 minerals are organic compounds. true false question 2 what vitamin can be found in foods like oranges, grapefruits, and broccoli? a. vitamin a b. vitamin k c.vitamin c d. vitamin d question 3 what are minerals? a. chemical elements that are needed for body processes. b. organic compounds that the body needs in small amounts to function properly. c. small molecules used to build proteins. d. an organic compound that is insoluble in water and includes fats. question 4 how many types of vitamins does the human body need? a. 15 b. 11 c. 13 d. 17 question 5 vitamins are a good source of energy. true false
Answers: 1

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
Questions


History, 16.02.2021 21:10

Mathematics, 16.02.2021 21:10

Mathematics, 16.02.2021 21:10

Arts, 16.02.2021 21:10

Advanced Placement (AP), 16.02.2021 21:10

English, 16.02.2021 21:10

Business, 16.02.2021 21:10


Mathematics, 16.02.2021 21:10


Chemistry, 16.02.2021 21:10

SAT, 16.02.2021 21:10


Mathematics, 16.02.2021 21:10

History, 16.02.2021 21:10


Mathematics, 16.02.2021 21:10

Chemistry, 16.02.2021 21:10

Mathematics, 16.02.2021 21:10

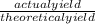 x 100%.
x 100%.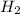 x
x 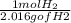 x
x 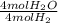 x
x 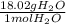 = 1.001 g
= 1.001 g 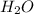
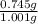 = 74.5%
= 74.5%

