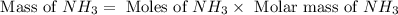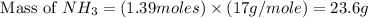Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How far is the region from the equator or control climate
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
When 100.g Mg3N2 reacts with 75.0 g H2O, what is the maximum theoretical yield of NH3?
M...
M...
Questions

Mathematics, 28.01.2020 06:31

Mathematics, 28.01.2020 06:31


Mathematics, 28.01.2020 06:31

Computers and Technology, 28.01.2020 06:31

Mathematics, 28.01.2020 06:31

English, 28.01.2020 06:31

Mathematics, 28.01.2020 06:31


Mathematics, 28.01.2020 06:31



Computers and Technology, 28.01.2020 06:31

Chemistry, 28.01.2020 06:31

English, 28.01.2020 06:31




Biology, 28.01.2020 06:31

History, 28.01.2020 06:31

 = 100.0 g
= 100.0 g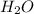 = 75.0 g
= 75.0 g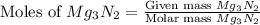
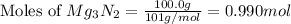
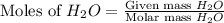
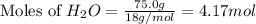
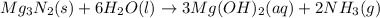
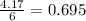 moles of
moles of 
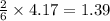 mole of
mole of