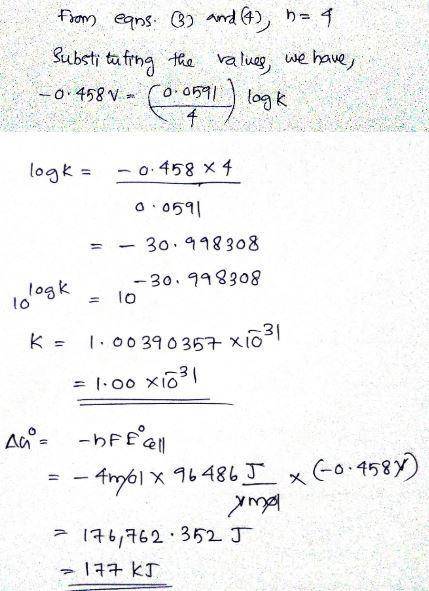Chemistry, 22.04.2020 21:00 Lydiaxqueen
Determine ∘ , Δ∘ , and K for the overall reaction from the balanced half-reactions and their standard reduction potentials. 2Co3+H3AsO3+H2O ↽−−⇀ 2Co2++H3AsO4+2H+Co3+e−↽−−⇀ Co2+ ∘=1.92H3AsO4+2H+2e−↽−−⇀ H3AsO3+H2O∘=0.575∘= ?K= ?Δ∘= ?Is the overall reaction spontaneous as written? not spontaneous spontaneousDetermine ∘E∘, Δ∘ΔG∘, and KK for the overall reaction from the balanced half-reactions and their standard reduction potentials.4Fe3++2H2O↽−−⇀ 4Fe2++O2+4H+Fe3++e−↽−−⇀ Fe2+ ∘=0.771 V1/2O2+2H++2e− ↽−⇀ H2O∘=1.229V∘=K=Δ∘=Is the overall reaction spontaneous as written?
A. spontaneous
B. not spontaneous

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
Determine ∘ , Δ∘ , and K for the overall reaction from the balanced half-reactions and their standar...
Questions





Business, 02.01.2020 17:31

English, 02.01.2020 17:31



History, 02.01.2020 17:31






Mathematics, 02.01.2020 17:31


History, 02.01.2020 17:31