
Chemistry, 22.04.2020 22:08 tdahna0403
Carbon monoxide and chlorine combine in an equilibrium reaction to produce the highly toxic product, phosgene (COCl2 ) CO(g) Cl2 (g) COCl2 (g) If the equilibrium constant for this reaction is Kc = 248, predict, if possible, what will happen when the reactants and product are combined with the concentrations shown. [CO] = [Cl2] = 0.010 M; [COCl2] = 0.070 M

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1
You know the right answer?
Carbon monoxide and chlorine combine in an equilibrium reaction to produce the highly toxic product,...
Questions

History, 20.10.2019 07:30



Health, 20.10.2019 07:30


Chemistry, 20.10.2019 07:30



Mathematics, 20.10.2019 07:30

Mathematics, 20.10.2019 07:30

Biology, 20.10.2019 07:30

Mathematics, 20.10.2019 07:30

Mathematics, 20.10.2019 07:30


Mathematics, 20.10.2019 07:30

Mathematics, 20.10.2019 07:30

English, 20.10.2019 07:30

English, 20.10.2019 07:30


Physics, 20.10.2019 07:30

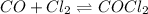
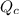 ) for this reaction is represented as:
) for this reaction is represented as:![Q_{c}=\frac{[COCl_{2}]}{[CO][Cl_{2}]}](/tpl/images/0619/3924/9faba.png)
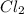 ] = 0.010 M and [
] = 0.010 M and [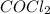 ] = 0.070 M
] = 0.070 M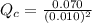 = 700
= 700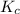 therefore reaction will proceed towards forward direction i.e. more
therefore reaction will proceed towards forward direction i.e. more

