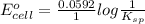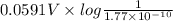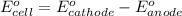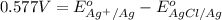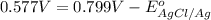Chemistry, 22.04.2020 22:03 Buttercream16
Calculate E ° for the half‑reaction, AgCl ( s ) + e − − ⇀ ↽ − Ag ( s ) + Cl − ( aq ) given that the solubility product constant for AgCl at 298 K is 1.77 × 10 − 10 and the standard reduction potential of the half‑reaction Ag + ( aq ) + e − − ⇀ ↽ − Ag ( s ) is + 0.799 V .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1


Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 2
You know the right answer?
Calculate E ° for the half‑reaction, AgCl ( s ) + e − − ⇀ ↽ − Ag ( s ) + Cl − ( aq ) given that the...
Questions



Mathematics, 29.10.2020 14:50


English, 29.10.2020 14:50

Chemistry, 29.10.2020 14:50

History, 29.10.2020 14:50

Mathematics, 29.10.2020 14:50

Mathematics, 29.10.2020 14:50

Mathematics, 29.10.2020 14:50

Mathematics, 29.10.2020 14:50


Mathematics, 29.10.2020 14:50

Biology, 29.10.2020 14:50


English, 29.10.2020 14:50


Mathematics, 29.10.2020 15:00


Mathematics, 29.10.2020 15:00

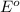 for the half-cell reaction is 0.222 V.
for the half-cell reaction is 0.222 V.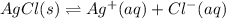
![K_{sp} = [Ag^{+}][Cl^{-}]](/tpl/images/0619/3632/031c0.png)
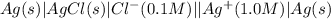
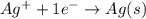 ,
, 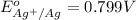
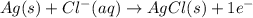 ,
, 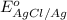 = ?
= ?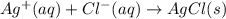
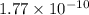
![E_{cell} = E^{o}_{cell} - \frac{0.0592 V}{n} log \frac{[AgCl]}{[Ag^{+}][Cl^{-}]}](/tpl/images/0619/3632/d09c5.png)
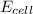 = 0.00 V
= 0.00 V![0.00 = E^{o}_{cell} - \frac{0.0592 V}{1} log \frac{1}{[Ag^{+}][Cl^{-}]}](/tpl/images/0619/3632/1250d.png)