
Benzene is a non-polar molecule, meaning that it does not have a positive or negative side. Which statement BEST describes how benzene will dissolve in water? A) Benzene can only exist as a gas and gases will not dissolve in water. B) Benzene dissolves well in water since polar molecules dissolve well in polar solvents. C) Benzene will dissolve in water because water is a universal solvent; it will dissolve anything. D) Benzene will not dissolve well in water since non-polar molecules do not dissolve in polar solvents.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 11:40
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 22.06.2019 17:00
Reduction is a reaction which results in a in electrons and a in positive charge of the atom or ion 1) a- loss 1) b- gain 2) a-increase 2) b-decrease
Answers: 1
You know the right answer?
Benzene is a non-polar molecule, meaning that it does not have a positive or negative side. Which st...
Questions

Mathematics, 02.02.2020 09:42

World Languages, 02.02.2020 09:42

Mathematics, 02.02.2020 09:42

History, 02.02.2020 09:42

English, 02.02.2020 09:42



History, 02.02.2020 09:42

English, 02.02.2020 09:42


Physics, 02.02.2020 09:42




Mathematics, 02.02.2020 09:42

Mathematics, 02.02.2020 09:42

Social Studies, 02.02.2020 09:42


History, 02.02.2020 09:42

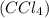 , e.t.c.
, e.t.c.

