
Chemistry, 22.04.2020 22:42 davi113001
The value of ΔG° at 181.0 °C for the formation of calcium chloride from calcium metal and chlorine gas is kJ/mol. At 25.0 °C for this reaction, ΔH° is -795.8 kJ/mol, ΔG° is -748.1 kJ/mol, and ΔS° is -159.8 J/K.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2


Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1
You know the right answer?
The value of ΔG° at 181.0 °C for the formation of calcium chloride from calcium metal and chlorine g...
Questions

Geography, 14.04.2020 05:52


Mathematics, 14.04.2020 05:52

Computers and Technology, 14.04.2020 05:52

Mathematics, 14.04.2020 05:52

Mathematics, 14.04.2020 05:52






Mathematics, 14.04.2020 05:53




Physics, 14.04.2020 06:10

English, 14.04.2020 06:11


Mathematics, 14.04.2020 06:11

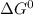 at 181.0
at 181.0 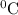 is -723.3 kJ/mol.
is -723.3 kJ/mol.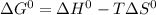
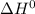 and
and 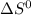 does not change in the temperature range 25.0
does not change in the temperature range 25.0 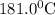 = (273+181.0) K = 454.0 K
= (273+181.0) K = 454.0 K ![\Delta G^{0}=(-795.8kJ/mol)-[(454.0 K)\times (-159.8\times 10^{-3}kJ/K.mol)]](/tpl/images/0619/5601/d1b5f.png)



