

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1
You know the right answer?
The true absorbance for a 1.0 x 10 −5 M solution is 0.7526. If the percentage stray light for a spec...
Questions








Physics, 04.02.2021 09:40

Biology, 04.02.2021 09:40

Mathematics, 04.02.2021 09:40

Chemistry, 04.02.2021 09:40

Mathematics, 04.02.2021 09:40




Mathematics, 04.02.2021 09:40

Mathematics, 04.02.2021 09:40



German, 04.02.2021 09:40

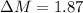 %
%
 %
% 
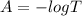
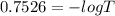

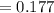
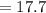 %
%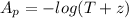
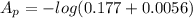
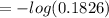
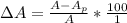
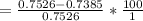
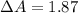 %
% 

