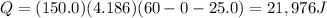CaO(s) + H2O(l) - Ca(OH)2(s)
enthalpy of rxn= -63.7 kJ/molrxn
Calcium oxide, CaO(...

Chemistry, 22.04.2020 23:16 ethangorrell67
CaO(s) + H2O(l) - Ca(OH)2(s)
enthalpy of rxn= -63.7 kJ/molrxn
Calcium oxide, CaO(s), has been proposed as a substance that can be used to heat water quickly for portable heating packs or for cooking. When placed in water, Cao(s) reacts as shown by the equation above.
A student wants to design a heating pad that could heat a 150.0 g sample of water from 25.0°C to 60.0°C.
Calculate the amount of heat, in joules, that the water must absorb for its
temperature to change by this amount. (Assume that the specific heat capacity
of the water is 4.18 J/gK).

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1
You know the right answer?
Questions




History, 31.05.2020 03:02


History, 31.05.2020 03:02

Mathematics, 31.05.2020 03:02

Computers and Technology, 31.05.2020 03:02


Computers and Technology, 31.05.2020 03:02








Mathematics, 31.05.2020 03:02


 , the amount of heat that must be supplied to the substance must be:
, the amount of heat that must be supplied to the substance must be: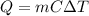
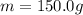 is the mass
is the mass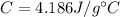 is the specific heat capacity of water
is the specific heat capacity of water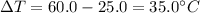 is the increase in temperature
is the increase in temperature