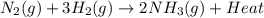Chemistry, 23.04.2020 00:08 shadowsnake
Ammonia is produced commercially by the Haber reaction: N2(8)+ 3H2(8)
The formation of ammonia is favored by
2NH3(8) + heat
an increase in pressure
a decrease in pressure
removal of N2(g)
4.
removal of H2(g)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
You know the right answer?
Ammonia is produced commercially by the Haber reaction: N2(8)+ 3H2(8)
The formation of a...
The formation of a...
Questions


Mathematics, 06.12.2019 04:31


Chemistry, 06.12.2019 04:31

History, 06.12.2019 04:31

Mathematics, 06.12.2019 04:31


English, 06.12.2019 04:31


Mathematics, 06.12.2019 04:31


Biology, 06.12.2019 04:31

Computers and Technology, 06.12.2019 04:31





Mathematics, 06.12.2019 04:31


English, 06.12.2019 04:31