

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3


Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1
You know the right answer?
The hydrolysis of tert-butyl chloride is given in the reaction below: (CH3)3CCl(aq) + H2O(l) → (CH3)...
Questions














Computers and Technology, 13.11.2019 05:31


Social Studies, 13.11.2019 05:31




Mathematics, 13.11.2019 05:31

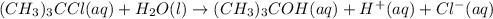
![\text{Rate}=k[(CH_3)_3CCl]^a[H_2O]^b](/tpl/images/0619/8048/0122d.png) ..........(1)
..........(1)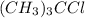
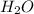
![\text{Rate}=k[(CH_3)_3CCl]](/tpl/images/0619/8048/db534.png) ...........(2)
...........(2)


