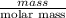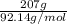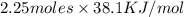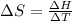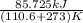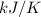Chemistry, 22.04.2020 23:45 phillipselijah2
The heat of vaporization Δ1, of toluene (C6H5CH3) is 38.1 kJ/mol. Calculate the change in entropy AS when 207. g of toluene boils at 1 10.6 °C. Be sure your answer contains a unit symbol. Round your answer to 3 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
The heat of vaporization Δ1, of toluene (C6H5CH3) is 38.1 kJ/mol. Calculate the change in entropy AS...
Questions

Spanish, 26.07.2019 13:50

Social Studies, 26.07.2019 13:50

Social Studies, 26.07.2019 13:50




Biology, 26.07.2019 13:50





Social Studies, 26.07.2019 13:50

Biology, 26.07.2019 13:50



History, 26.07.2019 13:50




Mathematics, 26.07.2019 13:50

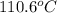 is 223
is 223 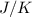 .
.