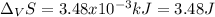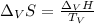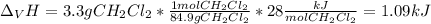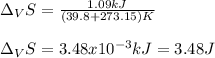Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1
You know the right answer?
The heat of vaporization ΔHv of dichloromethane CH2Cl2 is 28.0 /kJmol . Calculate the change in entr...
Questions



Mathematics, 21.12.2020 18:40

Physics, 21.12.2020 18:40



French, 21.12.2020 18:40





Mathematics, 21.12.2020 18:40

Mathematics, 21.12.2020 18:40

Mathematics, 21.12.2020 18:40

Computers and Technology, 21.12.2020 18:40



History, 21.12.2020 18:40