Chemistry, 23.04.2020 01:16 Delgadojacky0206
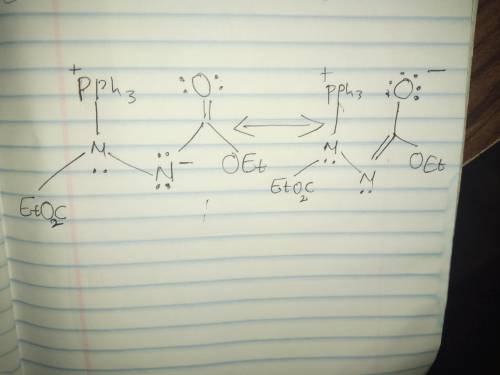
The Fischer esterification reaction synthesizes an ester via a nucleophilic acyl substitution reaction. The stereochemistry of the alcohol group is unchanged as it is the alcohol oxygen that makes the nucleophilic attack. The Mitsunobu reaction, on the other hand, allows the synthesis of an ester in which the stereochemistry of the alcohol is inverted. The reaction involves a carboxylic acid and an alcohol, as well as triphenylphosphine and an azo compound termed diethyl azodicarboxylate (DEAD). As with the Fischer esterification, H2O is formally lost during the reaction. However, in the Mitsunobu reaction the OH is lost from the alcohol and the H is lost from the carboxylic acid. The overall reaction is an SN2 reaction which occurs with inversion of configuration at the chiral carbon. Draw curved arrows to show the movement of electrons in this step of the mechanism.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
The Fischer esterification reaction synthesizes an ester via a nucleophilic acyl substitution reacti...
Questions

Chemistry, 23.09.2020 14:01



History, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01


Mathematics, 23.09.2020 14:01






Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01

Mathematics, 23.09.2020 14:01