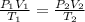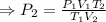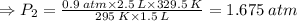Chemistry, 23.04.2020 01:12 lightning1157blaze
A sample of gas initially occupies 2.50 L at a pressure of 0.900 atm at 22.0°C. What will the pressure be if the temperature is changed to 56.5°C, and the volume is changed to 1.50 L?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
A sample of gas initially occupies 2.50 L at a pressure of 0.900 atm at 22.0°C. What will the pressu...
Questions

Spanish, 16.06.2021 15:20

Mathematics, 16.06.2021 15:20


Mathematics, 16.06.2021 15:20

Biology, 16.06.2021 15:20

Mathematics, 16.06.2021 15:20

Biology, 16.06.2021 15:20

History, 16.06.2021 15:20


History, 16.06.2021 15:20

Mathematics, 16.06.2021 15:20


Mathematics, 16.06.2021 15:30


Mathematics, 16.06.2021 15:30



Biology, 16.06.2021 15:30

Biology, 16.06.2021 15:30

Mathematics, 16.06.2021 15:30