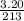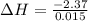Chemistry, 23.04.2020 02:56 LindaCat78
A "coffee-cup" calorimetry experiment is run for the dissolution of 3.20 g of aluminum nitrate placed into 103.2 mL of water. The temperature of the solution is initially at 23.2 oC. After the reaction takes place, the temperature of the solution is 17.7 oC. How much heat was absorbed or lost by the surroundings? Use 4.184 J/goC for the specific heat of the solution. Put your answer in units of kJ and make sure the sign is correct. What would be the enthalpy for the dissolution reaction of one mole of aluminum nitrate? Put your answer in kJ/mol and watch the sign for the enthalpy.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
A "coffee-cup" calorimetry experiment is run for the dissolution of 3.20 g of aluminum nitrate place...
Questions



Mathematics, 26.02.2021 17:10


Mathematics, 26.02.2021 17:10

Mathematics, 26.02.2021 17:10

Biology, 26.02.2021 17:10







Mathematics, 26.02.2021 17:10

Mathematics, 26.02.2021 17:10



Chemistry, 26.02.2021 17:10


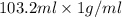
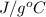 .
.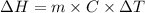
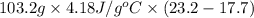
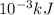 . So, 2372.5 J will be converted into kJ as follows.
. So, 2372.5 J will be converted into kJ as follows.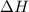 = 2.37 kJ
= 2.37 kJ 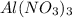 = 213 g/mol
= 213 g/mol