Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1
You know the right answer?
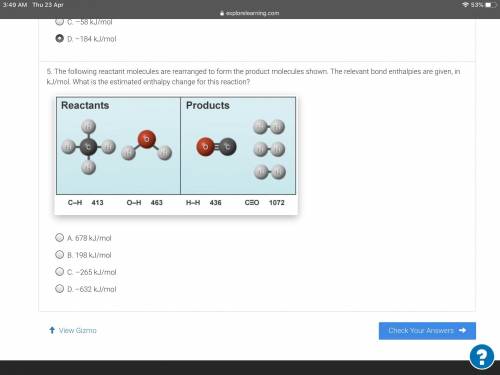
The following reactant molecules are rearranged to form the product molecules shown. The relevant bo...
Questions




Mathematics, 12.06.2020 02:57


Mathematics, 12.06.2020 02:57







Mathematics, 12.06.2020 02:57


Mathematics, 12.06.2020 02:57