Chemistry, 23.04.2020 03:38 christyr2002
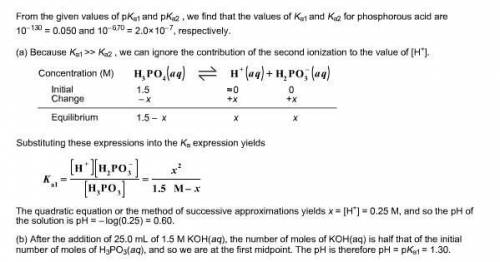
Phosphorous acid, H 3 PO 3 ( aq ) , is a diprotic oxyacid that is an important compound in industry and agriculture. p K a1 p K a2 1.30 6.70 Calculate the pH for each of the points in the titration of 50.0 mL of 1.5 M H 3 PO 3 ( aq ) with 1.5 M KOH ( aq ) . A molecule of phosphorous acid. A central phosphorus atom is single bonded to a hydrogen atom and two O H groups. An oxygen atom is also double bonded to the phosphorus atom.
a. before addition of any KOH :
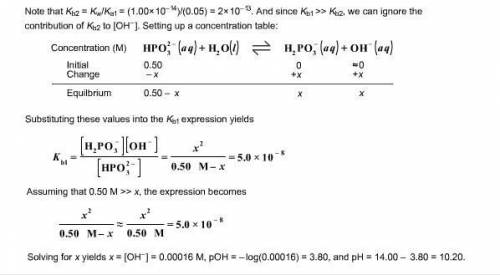
b. after addition of 25.0 mL KOH :
c. after addition of 50.0 mL KOH :
d. after addition of 75.0 mL KOH :
e. after addition of 100.0 mL KOH :

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3

Chemistry, 22.06.2019 11:00
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow.part ao2-(aq)+h2o(l)< => express your answer as part of a chemical equation. identify all of the phases in your answer.o2-(aq)+h2o(l) < => oh-(aq)+oh-(aq)part bpredict whether the equilibrium lies to the left or to the right of the equation in previous part.h2o is a stronger acid than oh–, so the equilibrium lies to the right.h2o is a weaker acid than oh–, so the equilibrium lies to the left.h2o is a stronger acid than oh–, so the equilibrium lies to the left.h2o is a weaker acid than oh–, so the equilibrium lies to the right.part cch3cooh(aq)+hs? (aq) < => express your answer as part of a chemical equation. identify all of the phases in your answer.ch3cooh(aq)+hs-(aq) < => h2s(aq)+c2h3o2-(aq)h2s(aq)+c2h3o2-(aq)part dpredict whether the equilibrium lies to the left or to the right of the equation in previous part.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the right.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the left.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the right.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the left.part eno2-(aq)+h2o(l) < => express your answer as part of a chemical equation. identify all of the phases in your answer.no2-(aq)+h2o(l) < => part fpredict whether the equilibrium lies to the left or to the right of the equation in previous part.hno2 is a stronger acid than h2o, so the equilibrium lies to the right.hno2 is a weaker acid than h2o, so the equilibrium lies to the left.hno2 is a stronger acid than h2o, so the equilibrium lies to the left.hno2 is a weaker acid than h2o, so the equilibrium lies to the right.
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
Phosphorous acid, H 3 PO 3 ( aq ) , is a diprotic oxyacid that is an important compound in industry...
Questions



English, 16.07.2019 10:00

History, 16.07.2019 10:00

Chemistry, 16.07.2019 10:00



English, 16.07.2019 10:00


Biology, 16.07.2019 10:00




Social Studies, 16.07.2019 10:00

Chemistry, 16.07.2019 10:00


History, 16.07.2019 10:00