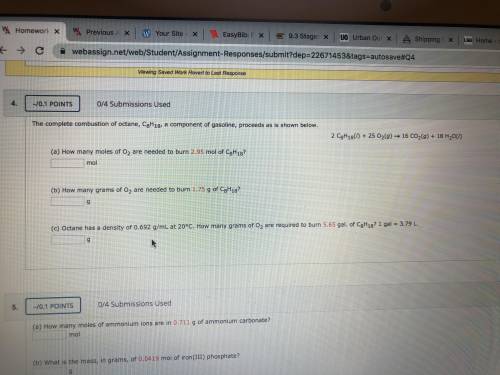
A) how many moles of O2 are needs to burn 2.95 mol of C8H18?
b) how many grams of O2 are...

Chemistry, 23.04.2020 03:22 avagymnast421
A) how many moles of O2 are needs to burn 2.95 mol of C8H18?
b) how many grams of O2 are needed to burn 1.75 g of C8H18?
c) Octane has a destiny of 0.692 g/mL at 20 degrees Celsius. How many grams of O2 are required to but. 5.65 gal. of C8H18? 1 gal= 3.79 L

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1
You know the right answer?
Questions

English, 04.10.2020 14:01

Mathematics, 04.10.2020 14:01


Mathematics, 04.10.2020 14:01


Health, 04.10.2020 14:01


Mathematics, 04.10.2020 14:01

History, 04.10.2020 14:01

Social Studies, 04.10.2020 14:01



Mathematics, 04.10.2020 14:01

Mathematics, 04.10.2020 14:01


Mathematics, 04.10.2020 14:01

Spanish, 04.10.2020 14:01


Mathematics, 04.10.2020 14:01



