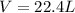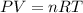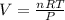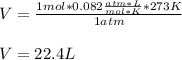Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3


Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
If 1 mol of gas is placed into a balloon under standard temperature and pressure (273 K and 1 atm),...
Questions


Mathematics, 18.12.2020 19:40

Mathematics, 18.12.2020 19:40





Mathematics, 18.12.2020 19:40


Mathematics, 18.12.2020 19:40

Mathematics, 18.12.2020 19:40


Mathematics, 18.12.2020 19:40

English, 18.12.2020 19:40

Mathematics, 18.12.2020 19:40


Mathematics, 18.12.2020 19:40


Law, 18.12.2020 19:40

Mathematics, 18.12.2020 19:40