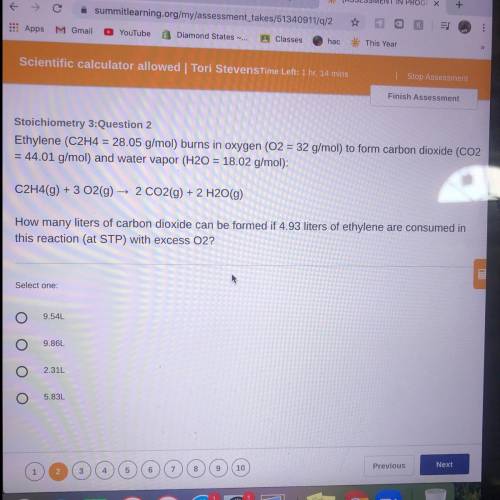
Ethylene (C2H4 = 28.05 g/mol) burns in oxygen (O2 = 32 g/mol) to form carbon dioxide (CO2
= 44...

Chemistry, 23.04.2020 20:23 tchase0616
Ethylene (C2H4 = 28.05 g/mol) burns in oxygen (O2 = 32 g/mol) to form carbon dioxide (CO2
= 44.01 g/mol) and water vapor (H20 = 18.02 g/mol):
C2H4(9) + 3 O2(g) → 2 CO2(g) + 2 H2O(g)
How many liters of carbon dioxide can be formed if 4.93 liters of ethylene are consumed in
this reaction (at STP) with excess O2?
Select one:
9.54L
9.86L
2.31L
5.83L

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1


Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Questions

Health, 07.12.2021 08:50

Mathematics, 07.12.2021 08:50

Mathematics, 07.12.2021 08:50






Mathematics, 07.12.2021 08:50

History, 07.12.2021 08:50

Mathematics, 07.12.2021 08:50



English, 07.12.2021 08:50


Mathematics, 07.12.2021 08:50



English, 07.12.2021 08:50



