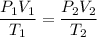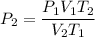Chemistry, 23.04.2020 22:23 christiantorres57
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point of -161. °C. Suppose the temperature of a sample of methane gas is raised from -13.0 °C to 17.0°C, and at the same time the pressure is changed. If the initial pressure was 0.26 kPa and the volume increased by 30.0%, what is the final pressure? Round your answer to the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
For many purposes we can treat methane (CH) as an ideal gas at temperatures above its boiling point...
Questions

Physics, 10.12.2021 03:00






Computers and Technology, 10.12.2021 03:00

Mathematics, 10.12.2021 03:00

Mathematics, 10.12.2021 03:00

Mathematics, 10.12.2021 03:00


Social Studies, 10.12.2021 03:00


Mathematics, 10.12.2021 03:00

Mathematics, 10.12.2021 03:00


Mathematics, 10.12.2021 03:00