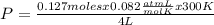Chemistry, 23.04.2020 23:47 isabel81ie
5.6 g of solid CO2 is put in an empty sealed 4.00 L container at a temperature of
300 K. When all the solid CO2 becomes gas, what will be the pressure in the
container? *
34.5 atm
O
O
0.78 atm
0.006 atrh
None of the other answers

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 09:00
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1
You know the right answer?
5.6 g of solid CO2 is put in an empty sealed 4.00 L container at a temperature of
300 K. When...
300 K. When...
Questions



Social Studies, 19.11.2019 09:31


Chemistry, 19.11.2019 09:31






Geography, 19.11.2019 09:31

History, 19.11.2019 09:31



Mathematics, 19.11.2019 09:31


English, 19.11.2019 09:31


Mathematics, 19.11.2019 09:31

History, 19.11.2019 09:31

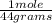 = 0.127 moles (being 44
= 0.127 moles (being 44 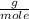 the molar mass of CO₂, that is, the amount of mass that a substance contains in one mole.)R= 0.082
the molar mass of CO₂, that is, the amount of mass that a substance contains in one mole.)R= 0.082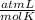 T= 300 K
T= 300 K