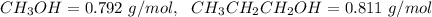Chemistry, 24.04.2020 01:00 robert7248
1. Calculate the number of moles of sulfuric acid that is contained in 250 mL of 8.500 M sulfuric acid solution
2. 7.300 moles of sodium nitrite are needed for a reaction. The solution is 5.450 M. How many mL are needed?
3. What mass (in g) of NH3 must be dissolved in 875 g of methanol to make a 0.430 molal solution?
4. Calculate the molality of a solution that is prepared by mixing 259.5 mL of CH3OH
(d = 0.792 g/mL) and 1387 mL of CH3CH2CH2OH (d = 0.811 g/mL)
5. A solution is prepared by dissolving 318.6 g sucrose (C12H22O11) in 4905 g of water. Determine the molarity of the solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
1. Calculate the number of moles of sulfuric acid that is contained in 250 mL of 8.500 M sulfuric ac...
Questions

World Languages, 03.10.2019 11:20

Spanish, 03.10.2019 11:20

Computers and Technology, 03.10.2019 11:20

Mathematics, 03.10.2019 11:20

History, 03.10.2019 11:20



Biology, 03.10.2019 11:20


English, 03.10.2019 11:20



Chemistry, 03.10.2019 11:20

Advanced Placement (AP), 03.10.2019 11:20

History, 03.10.2019 11:20



English, 03.10.2019 11:20


Geography, 03.10.2019 11:20