PLEASE HELP!
The following reaction was used to fuel the rockets in the Apollo mission l...

PLEASE HELP!
The following reaction was used to fuel the rockets in the Apollo mission landing module.
A) Is this reaction endothermic or exothermic?
B) How many grams of N2H4 must be reacted with an excess of N2O4 to produce 775 kJ of energy?
C) How many kJ of energy are produced when 6.25 g of N2O4 reacts with an excess of N2H4?
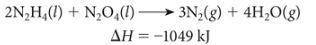

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Questions

Social Studies, 27.07.2019 10:00


History, 27.07.2019 10:00

English, 27.07.2019 10:00








English, 27.07.2019 10:00










