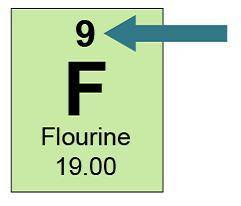
This is how fluorine appears in the periodic table.
A green box has F at the center and...

This is how fluorine appears in the periodic table.
A green box has F at the center and 9 above. Below it says fluorine and below that 19.00. A blue arrow points to 9.
What information does "9” give about an atom of fluorine? Select three options.
the atomic number
the atomic mass
the number of protons
the number of electrons
the number of neutrons

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
Questions

Mathematics, 21.02.2022 16:20



Mathematics, 21.02.2022 16:20

Computers and Technology, 21.02.2022 16:20



Mathematics, 21.02.2022 16:20


Mathematics, 21.02.2022 16:20


Mathematics, 21.02.2022 16:20


Social Studies, 21.02.2022 16:30

Chemistry, 21.02.2022 16:30




Computers and Technology, 21.02.2022 16:30

Mathematics, 21.02.2022 16:30



