
Chemistry, 08.10.2019 18:30 torresalysabeth
In a titration, 25.0 ml of 0.500 m khp is titrated to the equivalence point with naoh. the final solution volume is 53.5 ml. what is the value of v2 for the reaction?
a: 25
b; 0.5
c: 53.5
d: 28.5

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2

Chemistry, 22.06.2019 10:00
The tendency of water molecules to stick together is referred to as a) adhesion b) polarity c) cohesion d) transpiration e) evaporation
Answers: 1

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2
You know the right answer?
In a titration, 25.0 ml of 0.500 m khp is titrated to the equivalence point with naoh. the final sol...
Questions

Mathematics, 27.06.2019 18:30

Spanish, 27.06.2019 18:30


History, 27.06.2019 18:30

Mathematics, 27.06.2019 18:30

History, 27.06.2019 18:30


Mathematics, 27.06.2019 18:30

Mathematics, 27.06.2019 18:30

Mathematics, 27.06.2019 18:30


Computers and Technology, 27.06.2019 18:30

Health, 27.06.2019 18:30



Mathematics, 27.06.2019 18:30

Mathematics, 27.06.2019 18:30



Mathematics, 27.06.2019 18:30

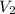 for the reaction is, (A) 28.5 ml
for the reaction is, (A) 28.5 ml

