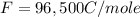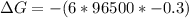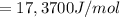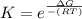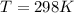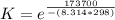Chemistry, 25.04.2020 03:33 madelynlittle5399
What is the value of the equilibrium constant at 25 oC for the reaction between the pair: Fe(s) and Cr3 (aq) to give Cr(s) and Fe2 (aq). Give your answer using E-notation with NO decimal places (e. g., 2 x 10-2 would be 2E-2; and 2.12 x 10-2 would also be 2E-2.). Do NOT include spaces, units, punctuation or anything else silly! Use the reduction potentials for Cr3 (aq) is -0.74 V and for Fe2 (aq) is -0.440 V. [a]

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

You know the right answer?
What is the value of the equilibrium constant at 25 oC for the reaction between the pair: Fe(s) and...
Questions












Mathematics, 07.04.2020 21:03








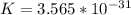
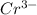 =
= 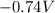
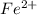 =
= 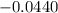
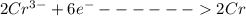
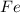 is oxidized to
is oxidized to 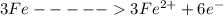
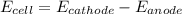
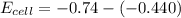
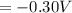
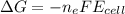
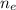 is the number of moles of electron which is 6
is the number of moles of electron which is 6