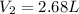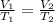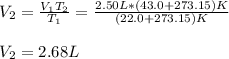Chemistry, 25.04.2020 02:59 Katepratt7637
Hot-air balloons rise because the hot air inside the balloon is less dense than the cooler air outside. Calculate the volume an air sample will occupy inside a balloon at 43.0C if it occupies 2.50L at the outside air temperature of 22.0C, assuming the pressure is the same at both locations

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

You know the right answer?
Hot-air balloons rise because the hot air inside the balloon is less dense than the cooler air outsi...
Questions

Biology, 11.11.2020 22:50

Mathematics, 11.11.2020 22:50



History, 11.11.2020 22:50

Social Studies, 11.11.2020 22:50


Spanish, 11.11.2020 22:50

Mathematics, 11.11.2020 22:50


Health, 11.11.2020 22:50

Mathematics, 11.11.2020 22:50


English, 11.11.2020 22:50


Mathematics, 11.11.2020 22:50

Mathematics, 11.11.2020 22:50


Physics, 11.11.2020 22:50

English, 11.11.2020 22:50