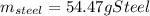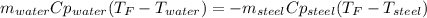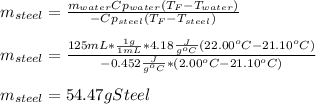Chemistry, 25.04.2020 03:20 kmarti11owj2q2
A volume of 125 mL of H2O is initially at room temperature (22.00 ∘C). A chilled steel rod at 2.00 ∘C is placed in the water. If the final temperature of the system is 21.10 ∘C , what is the mass of the steel bar? Use the following values: specific heat of water = 4.18 J/(g⋅∘C) specific heat of steel = 0.452 J/(g⋅∘C)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
A volume of 125 mL of H2O is initially at room temperature (22.00 ∘C). A chilled steel rod at 2.00 ∘...
Questions

Social Studies, 30.09.2019 17:20













Spanish, 30.09.2019 17:20

Mathematics, 30.09.2019 17:20


Mathematics, 30.09.2019 17:20

Social Studies, 30.09.2019 17:20