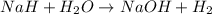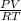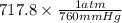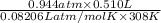Binary compounds of alkali metals and hydrogen react with water to liberate hydrogen gas. The hydrogen gas from the reaction of a sample of sodium hydride with an excess of water fills a volume of 0.510 L above the water. The temperature of the gas is 35 ∘C and the total pressure is 760 mmHg. Find the mass of H2 liberated and the mass of NaH that reacted.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
Binary compounds of alkali metals and hydrogen react with water to liberate hydrogen gas. The hydrog...
Questions



English, 05.07.2019 09:30

Biology, 05.07.2019 09:30

English, 05.07.2019 09:30

English, 05.07.2019 09:30






History, 05.07.2019 09:30



Mathematics, 05.07.2019 09:30


History, 05.07.2019 09:30

Advanced Placement (AP), 05.07.2019 09:30


English, 05.07.2019 09:30

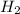 liberated is 0.0383 g and the mass of NaH that reacted 0.455 g.
liberated is 0.0383 g and the mass of NaH that reacted 0.455 g.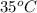 = (35 + 273) K = 308 K,
= (35 + 273) K = 308 K,