Chemistry, 25.04.2020 04:09 hellodarkness14
A piece of copper (12.0 g) is heated to 100.0 °C. A piece of chromium (also 12.0 g) is chilled in an ice bath to 0 °C. The specific heat capacity of water is 4.184 J/g ⋅°C.
Both pieces of metal are placed in a beaker containing 200.0 g at 20.0 °C.
(a) Will the temperature of the water be greater than or less than 20.0 °C when thermal equilibrium is reached?
(b) Both pieces of metal are placed in a beaker containing 200.0 g at 20.0 °C. Calculate the final temperature.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3

You know the right answer?
A piece of copper (12.0 g) is heated to 100.0 °C. A piece of chromium (also 12.0 g) is chilled in an...
Questions




English, 11.01.2020 23:31



Biology, 11.01.2020 23:31

Biology, 11.01.2020 23:31

Computers and Technology, 11.01.2020 23:31

Mathematics, 11.01.2020 23:31


History, 11.01.2020 23:31





English, 11.01.2020 23:31

Mathematics, 11.01.2020 23:31


Computers and Technology, 11.01.2020 23:31

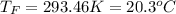
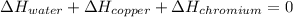
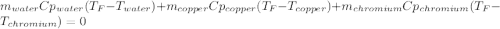 In such a way, by knowing that the heat capacities of copper and chromium are 0.386 and 0.45 J/(g°C) respectively, by solving for the equilibrium temperature one has:
In such a way, by knowing that the heat capacities of copper and chromium are 0.386 and 0.45 J/(g°C) respectively, by solving for the equilibrium temperature one has: