Titanium and chlorine react to form titanium(IV) chloride, like this:
TiCl3 → Ti(s)+ 2Cl2(g)<...

Chemistry, 25.04.2020 04:26 rachelbrooks764
Titanium and chlorine react to form titanium(IV) chloride, like this:
TiCl3 → Ti(s)+ 2Cl2(g)
At a certain temperature, a chemist finds that a 5.2L reaction vessel containing a mixture of titanium, chlorine, and titanium(IV) chloride at equilibrium has the following composition:
Compound Amount
TiCl4 4.18g
Ti 1.32g
Cl2 1.08g
Calculate the value of the equilibrium constant Kc for this reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2


Chemistry, 22.06.2019 23:50
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
Questions

Mathematics, 08.12.2020 23:30



Mathematics, 08.12.2020 23:30


Arts, 08.12.2020 23:30

Mathematics, 08.12.2020 23:30

Social Studies, 08.12.2020 23:30

English, 08.12.2020 23:30

Mathematics, 08.12.2020 23:30




Biology, 08.12.2020 23:30




Mathematics, 08.12.2020 23:30


Mathematics, 08.12.2020 23:30


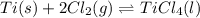
![Kc=\frac{1}{[Cl_2]^2}](/tpl/images/0626/7109/175a7.png)
![[Cl_2]_{eq}=\frac{1.08gCl_2*\frac{1molCl_2}{70.9gCl_2}}{5.2L}=2.93x10^{-3}M](/tpl/images/0626/7109/9b00a.png)



