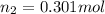A rigid tank contains 1.40 moles of an ideal gas. Determine the number of moles of gas that must be withdrawn from the tank to lower the pressure of the gas from 24.5 atm to 5.30 atm. Assume the volume of the tank and the temperature of the gas remain constant during this operation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
A rigid tank contains 1.40 moles of an ideal gas. Determine the number of moles of gas that must be...
Questions


Mathematics, 14.07.2020 01:01

History, 14.07.2020 01:01

Physics, 14.07.2020 01:01

Social Studies, 14.07.2020 01:01


Mathematics, 14.07.2020 01:01

English, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01


Geography, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01




Mathematics, 14.07.2020 01:01




Mathematics, 14.07.2020 01:01

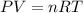
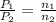
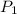 = initial pressure of gas = 24.5 atm
= initial pressure of gas = 24.5 atm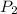 = final pressure of gas = 5.30 atm
= final pressure of gas = 5.30 atm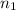 = initial number of moles of gas = 1.40 moles
= initial number of moles of gas = 1.40 moles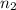 = final number of moles of gas = ?
= final number of moles of gas = ?