Chemistry, 25.04.2020 04:46 duttonsteven45
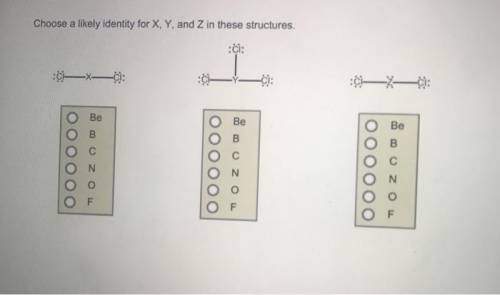
Choose a likely identity for X, Y, and Z in these structures. A central X atom is bonded to two chlorine atoms through single bonds. There are three lone pairs of electrons on each chlorine atom. C O B Be F N A central Y atom is bonded to three chlorine atoms through single bonds. There are three lone pairs of electrons on each chlorine atom. A central Z atom is bonded to two chlorine atoms through single bonds. There are three lone pairs of electrons on each chlorine atom and two lone pairs of electrons on the Z atom. C O N F Be B F C N Be B O

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3
You know the right answer?
Choose a likely identity for X, Y, and Z in these structures. A central X atom is bonded to two chlo...
Questions

History, 21.09.2021 14:00


Social Studies, 21.09.2021 14:00





Mathematics, 21.09.2021 14:00

English, 21.09.2021 14:00

English, 21.09.2021 14:00

Biology, 21.09.2021 14:00


Mathematics, 21.09.2021 14:00


Mathematics, 21.09.2021 14:00


Geography, 21.09.2021 14:00


Mathematics, 21.09.2021 14:00

Mathematics, 21.09.2021 14:00