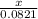Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

You know the right answer?
Ca(ClO3)2→ CaCl2 + O2
What mass of O2 results from the decomposition of 17.00 g of calcium ch...
What mass of O2 results from the decomposition of 17.00 g of calcium ch...
Questions

Mathematics, 12.11.2019 21:31


Mathematics, 12.11.2019 21:31


History, 12.11.2019 21:31

Biology, 12.11.2019 21:31



Social Studies, 12.11.2019 21:31

Mathematics, 12.11.2019 21:31


Social Studies, 12.11.2019 21:31

Biology, 12.11.2019 21:31



History, 12.11.2019 21:31

History, 12.11.2019 21:31

Spanish, 12.11.2019 21:31

English, 12.11.2019 21:31

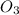 )
) ⇒
⇒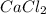 + 3
+ 3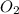
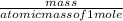
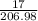
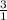 =
=