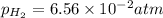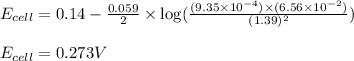Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
What is the calculated value of the cell potential at 298K for an
electrochemical cell with th...
electrochemical cell with th...
Questions

Mathematics, 09.02.2021 02:20



English, 09.02.2021 02:20



History, 09.02.2021 02:20




English, 09.02.2021 02:20



Mathematics, 09.02.2021 02:20





Spanish, 09.02.2021 02:20

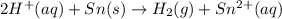
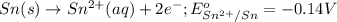
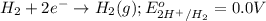
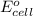 of the reaction, we use the equation:
of the reaction, we use the equation: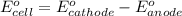
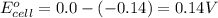
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Sn^{2+}]\times p_{H_2}}{[H^+]^2}](/tpl/images/0627/3491/76091.png)
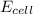 = electrode potential of the cell = ?
= electrode potential of the cell = ?![[H^{+}]=1.39M](/tpl/images/0627/3491/e38d3.png)
![[Sn^{2+}]=9.35\times 10^{-4}M](/tpl/images/0627/3491/dda0a.png)