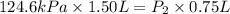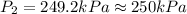Chemistry, 25.04.2020 23:17 WonTonBagel
A helium sample occupies 1.50 L of space at 124.6 kPa. What pressure would the helium need to experience to have a volume of 0.75 L?
A. 130 kPa
B. 97 kPa
C. 250 kPa
D. 62 kPa

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
A helium sample occupies 1.50 L of space at 124.6 kPa. What pressure would the helium need to experi...
Questions

Mathematics, 29.12.2019 02:31



History, 29.12.2019 02:31

Mathematics, 29.12.2019 02:31


Mathematics, 29.12.2019 02:31



Mathematics, 29.12.2019 02:31

Social Studies, 29.12.2019 02:31

Mathematics, 29.12.2019 02:31

History, 29.12.2019 02:31

Biology, 29.12.2019 02:31


English, 29.12.2019 02:31


Spanish, 29.12.2019 02:31


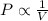
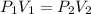
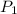 = initial pressure = 124.6 kPa
= initial pressure = 124.6 kPa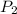 = final pressure = ?
= final pressure = ?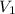 = initial volume = 1.50 L
= initial volume = 1.50 L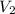 = final volume = 0.75 L
= final volume = 0.75 L