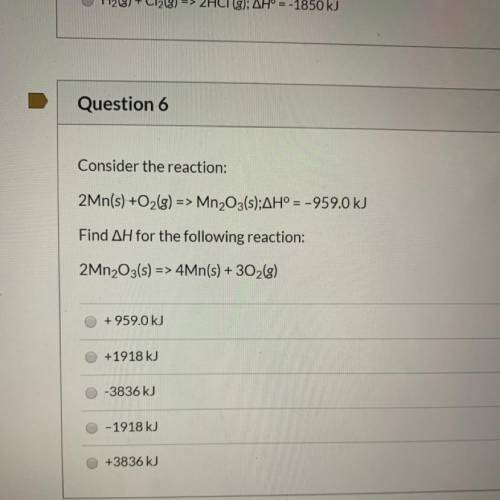
Consider the reaction:
2Mn(s) +O2(g) => Mn2O3(s);AH° = -959.0 kJ
Find AH for the foll...

Chemistry, 27.04.2020 01:29 CatsandDogsaredabest
Consider the reaction:
2Mn(s) +O2(g) => Mn2O3(s);AH° = -959.0 kJ
Find AH for the following reaction:
2Mn2O3(s) => 4Mn(s) + 302(g)
+959.0 kJ
+1918 kJ
-3836 kJ
O-1918 kJ
+3836 kJ

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3


Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

Chemistry, 23.06.2019 07:10
Which one of the following is an oxidation-reduction reaction? naoh + hno3 --> h2o + kno3 naoh + hno3 --> h2o + kno3 so3 + h2o --> h2so4 cacl2 + na2co3 --> caco3 + 2 nacl ch4 + 2 o2 --> co2 + 2 h2o al2(so4)3 + 6 koh --> 2 al(oh)3 + 3 k2so4
Answers: 3
You know the right answer?
Questions

History, 10.11.2020 17:00










History, 10.11.2020 17:00









Physics, 10.11.2020 17:00



