
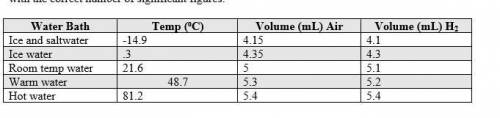
1. The actual value for absolute zero in degrees Celsius is −273.15. Use the formula below to determine your percent error for both gas samples.
|experimental value – actual value| x 100
actual value
2. If the atmospheric pressure in the laboratory is 1.2 atm, how many moles of gas were in each syringe? (Hint: Choose one volume and temperature pair from your data table to use in your ideal gas law calculation.)
Conclusion:
Write a conclusion statement that addresses the following questions:
• How did your experimental absolute zero value compare to the accepted value?
• Does your data support or fail to support your hypothesis (include examples)?
• Discuss any possible sources of error that could have impacted the results of this lab.
• How do you think the investigation can be explored further?

Answers: 3


Another question on Chemistry




Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1
You know the right answer?
1. The actual value for absolute zero in degrees Celsius is −273.15. Use the formula below to determ...
Questions




Mathematics, 02.08.2019 20:30


Mathematics, 02.08.2019 20:30


Social Studies, 02.08.2019 20:30

Mathematics, 02.08.2019 20:30


English, 02.08.2019 20:30

Biology, 02.08.2019 20:30

English, 02.08.2019 20:30

Mathematics, 02.08.2019 20:30


Biology, 02.08.2019 20:30

Mathematics, 02.08.2019 20:30


Mathematics, 02.08.2019 20:30



