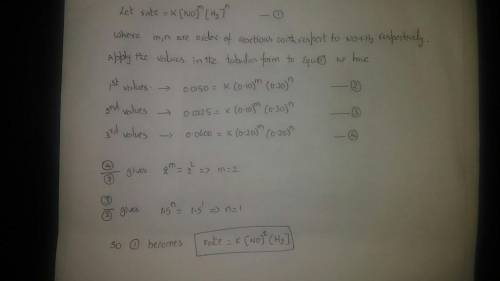Rate data have been determined at a particular temperature for the overall reaction 2NO(g) + 2H2(g) → N2(g) + 2H2O(g). The value of the specific rate constant at this temperature is ___.
Trial Run Initial [NO] Initial [H2] Initial Rate (M•s–1)
1 0.10 M 0.20 M 0.0150
2 0.10 M 0.30 M 0.0225
3 0.20 M 0.20 M 0.0600

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1


Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
Rate data have been determined at a particular temperature for the overall reaction 2NO(g) + 2H2(g)...
Questions




Chemistry, 24.06.2019 10:00





Mathematics, 24.06.2019 10:00

English, 24.06.2019 10:00



Business, 24.06.2019 10:00


Mathematics, 24.06.2019 10:00

Biology, 24.06.2019 10:00



History, 24.06.2019 10:00

English, 24.06.2019 10:00