
Suppose a 250.mL flask is filled with 0.70mol of H2 and 1.6mol of HCl. The following reaction becomes possible:
H2 (g) + Cl2 (g) <---> 2HCl (g)
The equilibrium constant K for this reaction is 0.262 at the temperature of the flask. Calculate the equilibrium molarity of HCl. Round your answer to two decimal places.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3


Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1
You know the right answer?
Suppose a 250.mL flask is filled with 0.70mol of H2 and 1.6mol of HCl. The following reaction become...
Questions


Mathematics, 11.12.2020 01:00


Health, 11.12.2020 01:00

English, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00

English, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00


Mathematics, 11.12.2020 01:00


Social Studies, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00

Mathematics, 11.12.2020 01:00





Engineering, 11.12.2020 01:00

Business, 11.12.2020 01:00

![\frac{[HCl]^2}{[H_{2}][Cl_{2}]}](/tpl/images/0648/3167/ae53b.png) = 0.262
= 0.262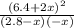 x = 0.285
x = 0.285 

